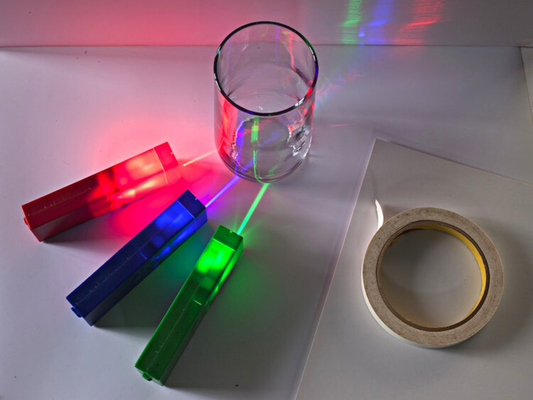
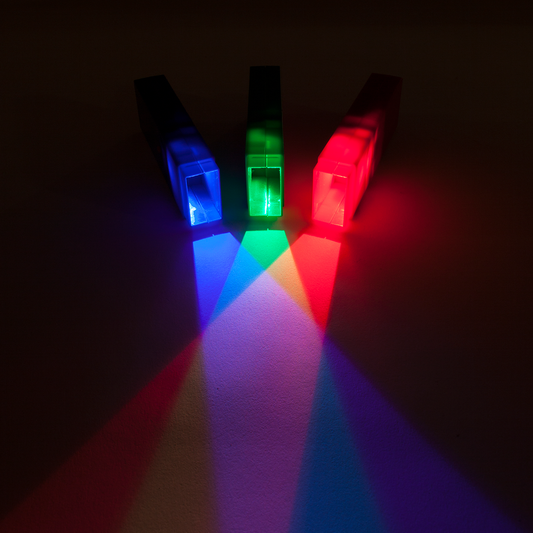
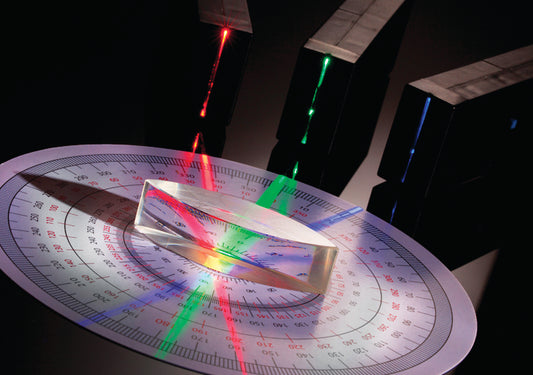
Safe laser pointers and optics educational kits
Safe classroom Laser Pointers in violet, green, and red!
We test every batch of laser pointers to ensure the output power is within the rated limits.
-
Violet Laser Pointer
Regular price $49.95Regular priceUnit price / per -
Classroom Green Laser Pointer
Regular price From $34.95Regular priceUnit price / per -
Super Safe Green Laser Pointer
Regular price $34.95Regular priceUnit price / per -
Dual Green and Red Laser Pointer (Switchable)
Regular price $49.95Regular priceUnit price / per -
Red Laser Pointer
Regular price $14.95Regular priceUnit price / per -
Super Safe Red Laser Pointer
Regular price $24.95Regular priceUnit price / per -
Acupuncture Cold Laser Therapy Pen
Regular price $39.95Regular priceUnit price / per -
LaserBlox Educational Laser Pointers - Ships August 2024
Regular price From $108.95Regular priceUnit price / per
Best-Seller for Classroom Use

Laser Classroom
Classroom Green Laser Pointer




Optics, Light, Lenses and Laser Educational Kits
Our educational products teach students all about color mixing with light, reflection, refraction, lenses, prisms, LEDs and lasers. See how colors and wavelength change the interactions and create fun rainbow experiments!
All kits and lessons are designed with extensive classroom testing and teacher feedback.
-
Light Blox - LED color mixing educational kit (Ships June 2024)
Regular price $29.95Regular priceUnit price / per -
Tech Light Lab LED Optics Kit - Ships July 2024
Regular price From $54.95Regular priceUnit price / per -
LaserBlox Educational Laser Pointers - Ships August 2024
Regular price From $108.95Regular priceUnit price / per -
All Morphed Up!
Regular price From $4.95Regular priceUnit price / per
The perfect kit for learning the fundamentals of light

Tech Light Lab LED Optics Kit - Ships July 2024
LASER CLASSROOM
Bringing STEM to Light for over 10 years
-

Trusted in classrooms
Our kits and tools have been used by thousands of students for over a decade, becoming the standard for education in light.
-
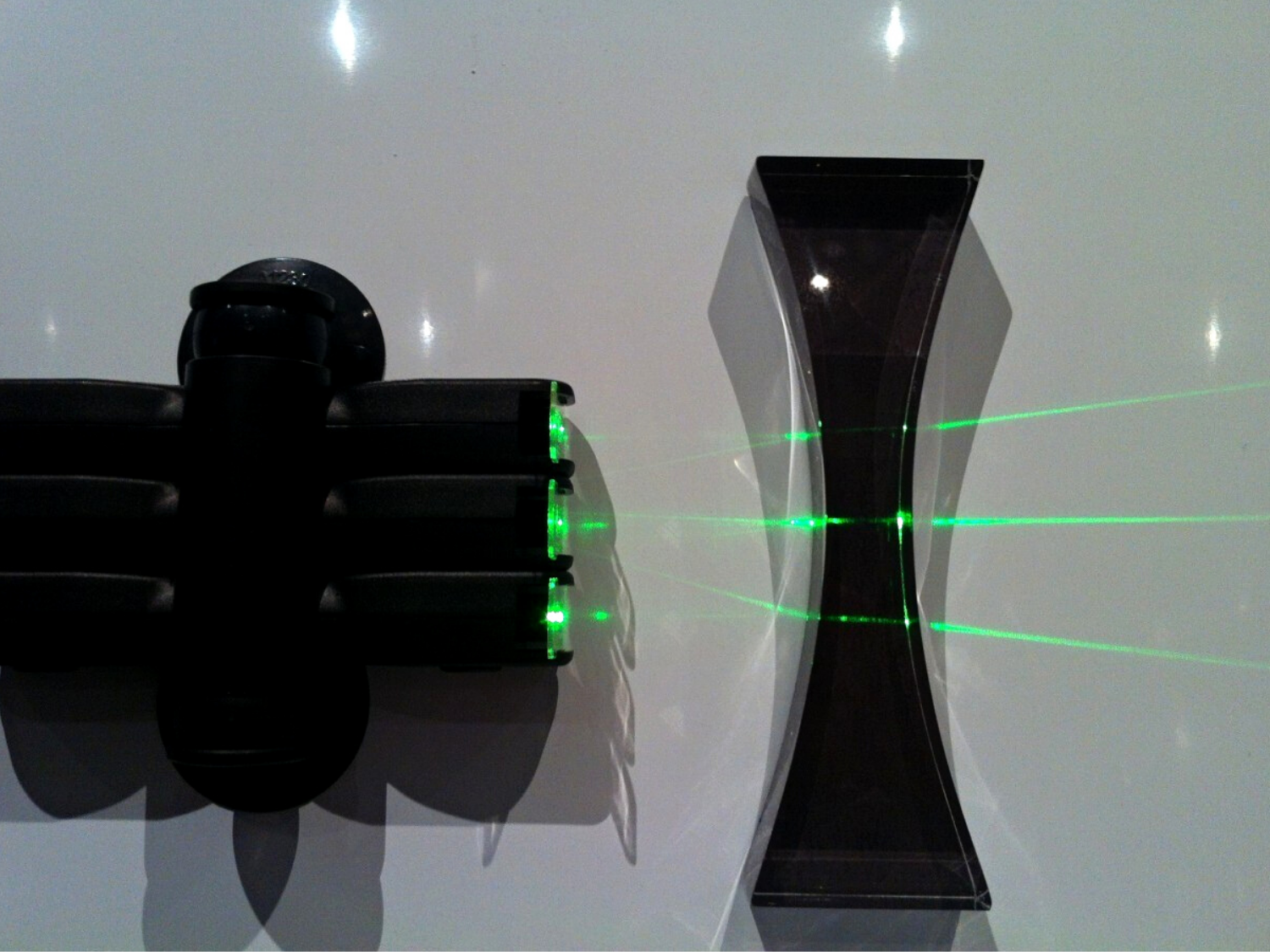
Designed by teachers
LaserBlox and LightBlox prototypes were extensively tested with teachers to perfect the design for real-world use.
-

SuperSafe Lasers
You don't always need the most powerful laser pointer to learn. Our SuperSafe Class IIA lasers are tested to ensure their output is FDA compliant.
Free lessons to learn about lasers, light, and optics.
View all-

Absorption and Transmission of Light (With Gumm...
Gummy Bears and lasers come together in this fascinating question, “What happens when you shine green light on a red gummy bear, or red light on a green gummy bear”?...
Absorption and Transmission of Light (With Gumm...
Gummy Bears and lasers come together in this fascinating question, “What happens when you shine green light on a red gummy bear, or red light on a green gummy bear”?...
-

Invisibility for Muggles!
Recent advances have brought us closer than ever to mastering one of the holy grails of science: invisibility. With an Invisibility Kit and this hands-on STEM activity uses a simple...
Invisibility for Muggles!
Recent advances have brought us closer than ever to mastering one of the holy grails of science: invisibility. With an Invisibility Kit and this hands-on STEM activity uses a simple...
Download free optics lessons
We'll email you a link to download the .ZIP file with over two dozen free PDF lessons!